- Have any questions? Contact us!
- info@dr-rath-foundation.org

Big Media: Not an Ally in the Campaign for Vitamin Freedom
July 27, 2009
Medycyna Komórkowa skuteczna ochrona zdrowia cz. 1
November 4, 2009Facts and Fiction About 2009 Swine Flu Pandemic

A global fear of swine flu pandemic that started in April of last year brought many important political and economic decisions affecting almost every country of the world. Was this threat real? Or was it a largely orchestrated campaign to take over and dominate the health care systems of many countries?
Read on to understand some important issues that should help in answering these questions.
Swine Flu – Reality of Its Occurence, Impact and Public Consequences
You can set your watch by it….
We are familiar with seasonal influenza (flu) which appears regularly during each winter or in early spring, when our bodies are weakened from staying indoors, little sun exposure, insufficient exercise and sub-optimal diets.
But in the last decade the dangers of global spread of viral infections have been appearing in the media quite regularly every 3 years. As such, about 6 years ago the media alerted us about the danger of the SARS pandemic, followed 3 years ago by bird flu, and now… a swine flu. Luckily these earlier pandemics did not spread beyond localized cases in a few countries. Perhaps, our public announcements presented in the New York Times, disclosing the real motives behind these threats, may have contributed to the abatement of fears.
Is swine flu pandemic a real threat?
If you rely solely on information in the media you can become easily confused. In fact, in May 2009 the World Health Organization (WHO) stated that the outbreak of swine flu had passed and that there was no immediate danger of its spread. However, a month later (June 2009), after a wave of various medical expert opinions surfaced predicting dire outcomes from swine flu virus, the WHO revised its statement and declared a swine flu pandemic (also called H1N1 2009 influenza). Of course, this put a lot of pressure on the governments of many countries to mobilize their resources and get ready to meet the challenge of combating the flu with drugs and vaccines.
The predicted numbers of potential victims of swine flu that were broadcast could send shivers down your spine. The White House council’s report said that this year alone the H1N1 virus could infect about 30 to 50 percent of the U.S. population, put 1.8 million into hospital and kill anywhere between 30,000 and 90,000 people.
When one looks at the facts, however, the reality is different. First, the virus seems to have a moderate impact. Although in the 4 months (April to August 2009) since its emergence, swine flu spread to about 168 countries and territories, the death cases amounted to no more than 1100 people. Every life is precious, but this is a tiny fraction compared to deaths occurring each winter from the seasonal flu which do not top global news reports and prompt governments into action. In addition, many doctors and public health officials agree that most cases of swine flu are mild and that most people recover from it fully (the death rate is about 0.03%). Second, as is the case with seasonal flu or other viral infections, there are some people who are more vulnerable to contracting the virus, or are at greater risk of developing health complications and even dying from it, than healthy individuals. These include small children, whose immune systems have not fully developed yet; people with impaired immunity, such as those suffering from other diseases; the elderly, especially those in nursing homes; people who are undernourished; and those with underlying illnesses.
Are flu statistics providing correct information?
- Reliability of swine flu case reports questionedDire predictions of swine flu are based on reports of H1N1 swine flu cases in different countries. The WHO, which collects these data, assures us that all reported flu cases have been confirmed in a laboratory as swine flu and that they are not regular or seasonal flu, which can give similar symptoms. However, at the same time, the WHO admits that in many instances the reports of swine flu are not confirmed, because there are no requirements to test for H1N1 2009 virus or even to report these cases any more. Aren’t you confused? I think everyone is. This means that the number of flu infections may be either higher or lower, and it can’t be excluded that common flu or other problems can be reported as swine flu.
- Seasonal flu deaths statistics under scrutiny:Problems with statistical data are not limited to those relating to newly emerged swine flu infections, as there have also been earlier concerns regarding the mortality statistics relating to regular seasonal flu.
According to official data, seasonal flu affects about 20% of the world’s population and accounts for about 500,000 deaths each year. The Center for Disease Control (CDC) states that in the US alone, every year about 36,000 people die as result of seasonal flu. (www.cdc.gov./flu/about/disease.htm).However, an article published in 2005 in the British Medical Journal questioned these mortality data (BMJ 10 December 2005, pg 1412). Its author disputes these statistics because they are not based on real data but on using a statistical model of “death-by-association”. According to this model, flu is always counted as a cause of death when it can be related to other medical causes of death. However, this “relation” does not specify any length of time between these events, and nor does it require testing for the presence of virus infections. Thus, by applying a “death by association” model, a person who died of pneumonia as a result of using anti-ulcer drugs can be counted as a flu death, because, at an unspecified time in life, he or she had a flu. Anti-ulcer drugs are not “associated” with pneumonia, although their use increases a risk of contracting it (JAMA 2004, 292, 1955-1960).A more thorough analysis published in 2003 in the Journal of the American Medical Association (JAMA) concluded that among 35,155 “flu” reported deaths every year, actually less than a quarter (8097) are caused by flu or flu associated pneumonia (JAMA 2003, 289, 179-1786). Therefore, death from “flu” may not in reality be from “flu” per se and statistical data are biased and need to be corrected.
- Does extrapolation of data from previous flu pandemics apply today?In many commentaries, the mortality from flu pandemic in 1918 is given as an example of what may happen with the spread of swine flu infections. However, based upon what has been observed so far, the severity of swine flu is not extremely high. In addition, such commentaries do not mention that the spread of 1918 flu may have been triggered and facilitated by war and existing living conditions such as a lack of access to clean water, nutrition and fresh air and an absence of antibiotics to treat bacterial infections stemming from flu. Simple hygiene and public health measures can limit the spread of infection more effectively that drugs or vaccines.
Focusing on swine flu pandemics distracts us from important health problems affecting most of the human population
The swine flu campaign is overshadowing other urgent problems that the people of the world face today and, moreover, is undermining scare financial resources that are needed to fight them. Poor living conditions, hunger and malnutrition affect people living in the developing world every day and are the breeding ground not only for swine flu but also many other viral, bacterial and parasitic diseases that cause millions of deaths. These diseases can be curbed not by new expensive pharmaceutical drugs, but by shifting financial resources from supporting pharmaceutical multinationals to improving living conditions, access to clean water, nutritious food and supplements, education and applying other simple, natural and cost effective measures.
Only about $300 million is needed to eliminate deficiency of iron or vitamin A in the developing world. These micronutrients are essential for immune system function and can be provided by micronutrient supplementation or the enrichment of food. The cost of this project would represent a small fraction of the world pharma market – which in 2008 accounted for $773 billion. Much of it was made by selling ineffective drugs to poor nations suffering mostly from malnutrition and vitamin deficiencies.
Threat of a Pandemic Turns on Green Light for Drug Business
The perceived threat of a swine flu epidemic resulted in urgent action from public health officials aimed at implementing preventive strategies and making vaccines and anti-viral drugs available to large populations of people. As such, governments have been stockpiling medical drug supplies and relaxing regulations for new swine flu vaccines. Already, various legal constraints have been removed allowing many governments to implement “fast-track” approaches in testing anti-flu vaccines and dispensing various anti-viral drugs, thus turning a blind eye to their safety and the adjusting of proper doses. At the same time, warnings have been issued against promoting vitamins and other natural remedies against swine flu.
Legal immunity for drug makers: As one of the “emergency” measures, the US Health and Human Services Secretary, Kathleen Sibelius, has given legal protection to the makers of Tamiflu and Relenza for any claims regarding injuries caused by these drugs. In addition, as published in the June 25, 2009 Federal Register, she granted legal immunity to the manufacturers of the future swine flu vaccines and any adjuvants they contain.
With this regulation in place, pharmaceutical firms have no financial incentive to make the safest product. Even more, the less they know about any safety problems the products may pose, the better they protect themselves. Therefore, they are not interested in extensive testing of their products for harmful effects, for, if they do not know about them, they are not liable.
Take drugs NOT vitamins!: At the same time, a very strong warning has been issued against the manufacturers of nutritional supplements, prohibiting them from issuing any health claims regarding their products and swine flu. This includes many natural substances supported by clinical and scientific evidence of their effectiveness in suppressing influenza virus, as well as strengthening the natural function of the immune system – our main protection and defense against viral and other infections. This topic is addressed in more details below.
Fear of Pandemic – Effective Measure to Strangulate Economies of Many Countries
The financial costs associated with preparations to fight the pandemic are enormous. Since 2004, the US government paid pharmaceutical companies about $7.9 billion to develop the capacity to mass vaccinate the entire US population by 2011. This plan included both setting up the production capacity to supply vaccines to the entire US population within 6 months, and stockpiling enough vaccines to inoculate 20 million people soon after the onset of an epidemic. Currently, an additional $9 billion has been assigned to fight swine flu.
The financial cost of stockpiling vaccines is also high. There are about 22.5 million doses of H5N1 bird flu antigen that were stockpiled for an anticipated pandemic that never materialized. The cost of maintaining just two circulating bird flu strains of H5N1 is about $2.2 billion every year. Since flu vaccine expires after 2 years, about 15 million doses of it have already expired or will expire soon.
All this is a high burden on an already sick US economy, but it has a much bigger impact on the financial resources of most of the countries in the developing world. An orchestrated PR effort in spreading fears among people allows the pharmaceutical multinationals to expand their business and keep even good-willed politicians hostage to the pharma dictatorship.
Anti-Flu Drugs and Vaccines Carry Health Risks
Are anti-viral drugs effective and safe against swine flu?
Currently, four pharmaceutical drugs are recommended for flu treatment and to prevent virus infections: the adamantanes (Amantadine and Rimantadine) and a newer class of neuraminidase inhibitors (zanamivir – Relenza, and oseltamivir – Tamiflu).
- Adamantanes are not effective against the swine flu virus, and, when used against other influenza A type infections (ie seasonal flu), were associated with toxic side effects and development of drug-resistant viruses.
- Tamiflu and Relenza block the function of a viral protein, neuraminidase, which is needed for a virus to leave the cells and spill into surrounding lung tissue. These drugs have to be administered very early (within 12 hours to 2 days) in order to expect any effects. However, efficacy of these drugs has not been impressive: studies with Tamiflu conducted in children and the elderly showed a reduction of in the length of illness of only 1 day. These drugs are not approved for children younger than 1 year old or for pregnant women.
The side effects of Tamiflu mostly include nausea and vomiting. Other adverse reactions have been identified based on the reports from post-marketing use of this drug. These reports are only voluntary and include many unknown aspects, therefore the frequency of occurrence of these side effects is not certain. These effects include swelling of the face or tongue, allergy, rash, hepatitis, abnormal liver tests, arrhythmia, seizures, aggravation of diabetes and many others. Toxicity, side effects and the development of drug resistance therefore argue against the use of these drugs for flu prevention.
There are limited data regarding the effectiveness of these four antiviral agents in preventing serious influenza-related complications (e.g., bacterial or viral pneumonia or exacerbation of chronic diseases), because they were tested mainly on patients with uncomplicated flu infections. It is not known how effective they are in people who are at high risk for serious complications from influenza. Even fewer studies with these drugs were conducted among children, especially children younger than1 year. It is also not known whether they can suppress the spread of virus from person to person.
Exposing patients to more health risks from inadequately tested drugs
Tamiflu and Relenza can cause many side effects when taken orally, but now their manufacturers (Roche and GlaxoSmithKline) are pressing the U.S. Food and Drug Administration (FDA) to quickly approve new, intravenous formulations of these drugs. These decisions will also include consideration of a new experimental drug, Peramivir, manufactured by BioCryst Pharmaceuticals. By calling the H1N1 pandemic “a serious threat to our nation” it is likely that they will succeed.
This means that if intravenous delivery of these drugs is approved without proper safety and efficacy testing, it can put many people at risk, especially so given that these drugs will be given mainly to those who are seriously ill. According to the product description for an oral form of Tamiflu, the effects of this drug are not known “in patients with any medical condition sufficiently severe or unstable to be considered an imminent risk of requiring hospitalization”. It is therefore quite possible that intravenous delivery of these drugs to already ill people may endanger their lives even more so than would the flu itself.
Accelerating impact of drug side effects
Supplying anti-viral drugs to a very large number of people, such as in the threat of a pandemic, would magnify the risks of adverse side effects – especially so given that swine flu seems relatively mild (death rate about 0.03%) and that the immune systems of the majority of people can overcome this infection. Also, the widespread use of these drugs could lead to the development of resistant strains – this is already the case with a human influenza H1N1 subtype, which in the past few years has become almost completely resistant to Tamiflu. (Science 2009; 323 (5918): 1162-1163). Also, it should be noted that influenza A viruses resistant to anti-viral drugs adamantine and rimantadine can emerge very quickly – as early as 2-3 days after initiation of drug therapy. (Curr Top Microbiol Immunol 1992; 176: 119-130)
How vaccines work in our body
Vaccines are being used to boost the body’s immune system to better attack infectious agents such as viruses. They traditionally contain a virus (antigen) that has been weakened or killed, as a trigger to the generation of a protective immune response. In addition, some vaccines contain other immune boosting substances, known as adjuvants (in Latin “to help”) as well as preservatives.
Vaccines are intended to stimulate the immune system without doing serious harm to the host, so that when the real pathogen infects a person, the immune system responds to it quickly and blocks its spread. However, vaccines have a very limited use in treating patients who have already been infected. They are also largely ineffective against rapidly mutating viruses, such as influenza (this is why seasonal flu vaccines are updated every year).
How the body responds to vaccination
Since the main purpose of vaccination is to trigger an immune system response, there are many adverse effects that can develop. These are separated into two types: local and general reactions.
- Local reactions range from pain at the injection site to inflammation, redness, swelling, abscess formation, or ulceration. These are transient effects and should not last longer than a few days.
- Vaccine reactions that affect the entire body may include nausea, fever, development of adjuvant related arthritis, allergic reactions, organ-specific toxicity, anaphylaxis, immunosuppression, and induction of autoimmune diseases. (Allison AC, Byars NE. Immunological adjuvants: desirable properties and side-effects. Mol Immunol 1991; 28(3): 279–284; Waters RV, Terrell TG, Jones GH. Uveitis induction in the rabbit by muramyl dipeptides. Infect Immun 1986; 51(3): 816–825).
While some general reactions such as allergy and anaphylaxis can be triggered by the antigen, others, such as adjuvant arthritis, may be caused directly by or exacerbated by the adjuvant. It can therefore be difficult to identify which adverse reactions are mediated by the antigen, which by the adjuvant, and which by both.
Why adjuvants are used in vaccines
Adjuvants are added to boost immune response while using smaller amount of viral antigen. This allows for stretching the supplies of an antigen to produce more doses and sell more products. However, adjuvants can generate additional health complications and side effects.
In case of threat of serious diseases, antigen–sparing approaches can benefit people by making more vaccines available; however, at the same time, this also increases the health risks to individuals. In the case of an anticipated swine flu pandemic – which, so far, has had a mild health impact – this massive push for vaccination of almost the entire population looks more like it is motivated by “business profits” rather than our health concerns.
Dangers of adjuvants
The adjuvants considered to be used in swine flu vaccines include Alum and Squalene. Both of these substances can trigger serious adverse reactions in our body. Currently, whilst some vaccines have been made with squalene adjuvants, some will not contain any.
- Aluminum hydroxide/phosphate formulation, known as Alum
Alum adjuvants are FDA-approved, but their exact mechanism of action is not well known. Researchers have found that aluminum delivered in a vaccine together with an antigen (ie, killed virus) becomes engulfed by the white blood cells (macrophages). Since aluminum is toxic it triggers the death of these white blood cells, which, as a result, releases various inflammatory mediators in the lymph nodes. Consequently, a mixture of macrophage cell debris, antigen and inflammatory factors stimulates an antibody response and leads to the development of immunity against the virus.Unfortunately, despite being FDA-approved, alum adjuvants have significant adverse effects, including chronic inflammation syndrome (MMF), causing problems such as myalgia, muscle weakness, and fever. In some patients there were reports of neurological abnormalities, in addition to pain at the injection site, inflammation, or sterile abscess. (Goto N, Kato H, Maeyama J, Eto K, Yoshihara S. Studies on the toxicities of aluminum hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine 1993; 11(9): 914–918) Although many adjuvant-caused vaccine reactions are not life-threatening, their long-term adverse effects have been implicated in the development of allergies and autoimmune diseases.Aluminum is being commonly used in human vaccines and children can receive up to 3.75 mg of parenteral aluminum during the first 6 months of life. Research has shown that injection of aluminum-adsorbed vaccines in mice caused a transient increase of aluminum level in the brain; therefore there are concerns regarding the accumulation of this metal in brain tissue and a risk of Alzheimer’s disease later in life. - Squalene adjuvants:
Squalene is a naturally occurring substance that is produced by the liver to help metabolize cholesterol and combat physical injuries. It is also delivered to our body in foods such as fish liver oil, some vegetable oils, various nutritional supplements or by external application in some cosmetics.However, since the 1980s, squalene has been studied by the National Institutes of Health to be used as an adjuvant to boost the efficacy of certain vaccines. It can stimulate immune system response, but, because animal studies have shown that squalene adjuvants may generate unwanted side effects – including autoimmune versions of arthritis, multiple sclerosis and other conditions – the FDA has never approved the general use of any vaccine containing squalene in the United States.Only 3 vaccines using these adjuvants (MF59 (Novartis) and ASO3 (Glaxo Smith-Kline)) have been licensed in Europe. In 1997, European health agencies approved emulsified squalene (with influenza virus in the center of each droplet) for use as an adjuvant in an influenza vaccine (Fluad, Chiron Corporation, Marburg, Germany, and Siena, Italy; Sesardic & Dobbelaer, 2004). Although Novartis claims that its adjuvant has been used safely by about 40 million people, the FDA did not approve even a single US vaccine using it – until now.
Dangers of adjuvants: lessons from history
In the 1976 flu outbreak about 40 million people were vaccinated with an inadequately tested vaccine against swine flu (containing squalene). Serious health complications affecting the nervous system that vaccinated people subsequently developed, a condition known as Guillain Barre Syndrome (GBS), resulted in the termination of the campaign and withdrawal of the vaccine.
Safety of squalene as vaccine adjuvant was also called into question when a professor at Tulane University conducted a study of more than 400 Gulf War veterans suffering from Gulf War Syndrome (GWS) – a condition characterized by general fatigue, joint pain, memory loss, an inability to concentrate, and other ailments. Ninety-five percent of those who exhibited symptoms of GWS had high levels of squalene antibodies in their blood. Many of the samples that tested positive for squalene were taken from soldiers who were not deployed to the Persian Gulf but still received the anthrax vaccine containing squalene as an adjuvant. However, compared to the minimal amounts of squalene measured in 5 lots of anthrax vaccine in 2000, current squalene-containing adjuvants are likely to provide many orders of magnitude more squalene per vaccine dose. No one knows how much of this substance it takes to cause adverse effects, what kind, or in which populations.
Today, as in 1976, official concern is with the availability of the flu vaccine, without regard for its safety. Aren’t we learning from history?
Will we ever know the truth?
Tests of a swine vaccine with new adjuvants in people are very short (about 4 weeks) before mass vaccination begins. Yet autoimmune side effects take months to appear; for instance, the manifestation of GBS took 4 -8 weeks. In addition, the potential variety of adverse reactions to the vaccine is very large. As such, it will be difficult to sort out quickly which of them are vaccine related before tens of millions of people are vaccinated.
In the UK, it seems that health services are cognizant of these risks as they have set up a surveillance system (about 600 letters to neurologists were sent in August this year) to track down and immediately report cases of GBS. Unfortunately, however, the risk does not begin and end with GBS.
We are not aware of health surveillance systems operating in connection with the new flu vaccines in other countries. On September 15th, the FDA accepted 4 new flu vaccines that will be available by around mid-October; primarily for vaccinating pregnant women, infants, children and people with existing diseases. Pressure for vaccinations is building up. It is still voluntary, but there are steps already in place to make swine flu vaccination mandatory. There is still time to act to prevent it if you are concerned with your health and the health of your loved ones.
General Information About Swine Flu and the Virus Causing It
How can I recognize whether I have swine flu?
The swine flu has the same symptoms as seasonal influenza: fever, cough, muscle aches, sore throat, sometimes intestinal problems, and others. Usually these symptoms last for a few days, sometimes longer than a week, and then our body recovers. Infection by a swine flu virus can be confirmed after laboratory testing for the type of virus present in the body. Compared to seasonal flu, it seems that swine flu virus retains its infectivity for longer (about a week, compared to 2-3 days). In general, the disease itself is relatively mild, and, just as with seasonal flu, the majority of people can overcome it by taking plenty of rest, drinking lots of fluids, and following a light diet supplemented with vitamins and other essential nutrients. READ MORE.
Who is at risk of complications from swine flu?
Swine flu has a greater impact on people with a compromised immune system, such as the elderly; small children; those suffering from other diseases (cancer, AIDS); those taking immuno-suppressive drugs (be aware here that recent research suggests cholesterol-lowering drugs – Statins – can suppress the immune system); people who are malnourished or excessively dieting; and those suffering from blood disorders or micronutrient deficiencies. In such cases, flu can substantially weaken the body making it prone to bacterial infections (pneumonia) or developing other health problems.
It is well known that infections increase the need for vitamin C. It has been shown that in response to infection, white blood cells actively start accumulating this vitamin; the resulting intracellular concentrations of which increase more than 10 times. People suffering from flu or other infections are often deficient in vitamin C, which may contribute to an increased risk of heart attacks, asthma and other problems.
This is why all natural means of strengthening our immune system – such as good nutritious food, nutrient supplementation, clean environment, fresh air, enough sleep and physical exercise – are critical in the effective prevention and recovery from flu and other viral infections. Specific vitamins and other nutrients are necessary to naturally curb the biochemical processes that control cell infectivity, viral multiplication and the spread of the virus in the body.
Micronutrient Effectiveness Against Influenza
Alternatives to toxic anti-viral drugs do exist. For example, several nutrients have been shown to modulate the immune system as well as to suppress the replication and spread of viruses.
Several nutrients are essential for optimum function of the immune system. These include: vitamins C, A, D, E, B6, B12, folic acid, iron, zinc, copper and iodine for cell mediated immunity (optimal functioning of leukocytes, macrophages/monocytes, T-cells and thymus); and vitamins A, D, B1, B2, B3, B6, pantothenic acid, biotin, folic acid, zinc and copper for optimal antibody production (humoral immunity).
There is substantial scientific evidence that vitamin C, amino acids (lysine, proline, arginine), N-acetyl cysteine (NAC) and EGCG (from green tea extract) can suppress virus multiplication or viral spread in the tissue.
Flu virus can be controlled at several levels by nutrients:
- directly, by affecting the metabolism of specific viruses and thereby limiting their ability to multiply and infect the cells, and
- indirectly, by interfering with cell-to-cell spread of viruses in the tissue.
Our Institute’s approach to the control of influenza has focused on the use of naturally-derived substances with antiviral potential. As one effective strategy in fighting influenza, we have focused specifically on the action of micronutrients and their synergy in controlling the infection and spread of human influenza virus, without imposing toxic effects on normal cells of the host. This research direction was triggered by Dr Rath’s work published in 1992.
This approach has been shown to be successful in confirming the effectiveness of nutrient combinations against human influenza A virus (subtype H1N1). The salient findings from our research are summarized below.
Micronutrient synergy in natural control of H1N1 virus infection
The main aim of our study was to evaluate the effectiveness of a unique micronutrient mixture, containing ascorbic acid, green tea extract, selected amino acids, selenium and other nutrients against human influenza A (H1N1) virus with respect to its actions on
- activity of a virus-associated enzyme (neuraminidase) required for infectivity;
- production of a viral core protein (nucleoprotein, NP), an indicator of viral replication, and
- induction of biological responses essential for extra-cellular invasion of viruses. These include secretion of enzymes digesting connective tissue (metalloproteinases) and viral invasion in the extracellular environment, indicative of its spread. (Fig 1)
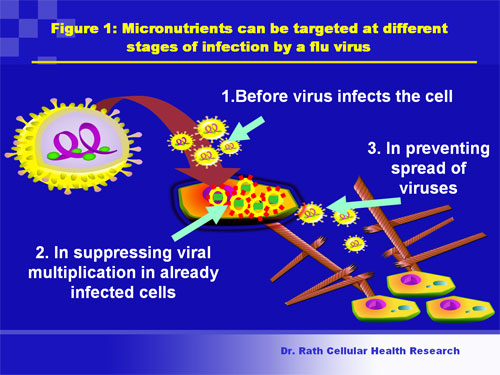
Figure 1. Steps in influenza infection: Micronutrients can be targeted at multiple steps in the infection of host cells by the flu virus.
The components of the micronutrient mixture were chosen based on their prior reported inhibitory activity towards the replication of the influenza virus and HIV, as well as their ability to inhibit the growth and/or spread of cancer cells.
The results obtained showed that incubation of isolated H1N1 virus with the nutrient mixture resulted in dose-dependent inhibition of neuraminidase enzymatic activity in viral particles (Fig 2) and reduction of viral infectivity upon infection of host cells.
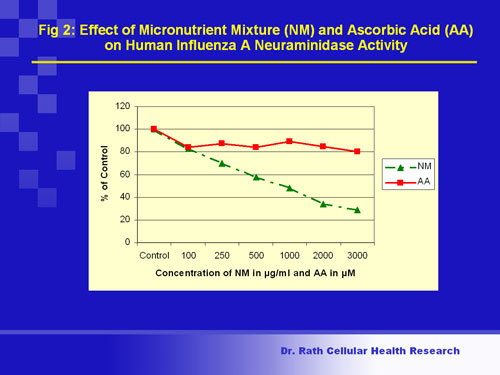
Fig. 2
Figure 2. Viral infectivity (enzyme activity): Flu viruses have on their surface a protein (enzyme) called neuraminidase. The neuraminidase enzyme enables a virus to detach from infected host cells and infect other cells. Inhibiting or lowering this activity can make viruses ineffective. The figure shows that incubation of cell-free virus with the micronutrient mixture inhibited the viral neuraminidase activity in a dose-dependent manner, causing 70% inhibition at the highest concentration of the nutrient mixture. In contrast, a single nutrient (ascorbic acid or vitamin C) produced only 20% inhibition at its maximal dose, suggesting cooperative inhibitory effects of the nutrients in the mixture.
Conclusion: Nutrients can cooperatively inhibit neuraminidase enzyme activity associated with human influenza A (H1N1) virus by 70%.
Application of this nutrient mixture to host cells after their infection with the virus led to dose-dependent suppression of viral replication. (Fig 3)
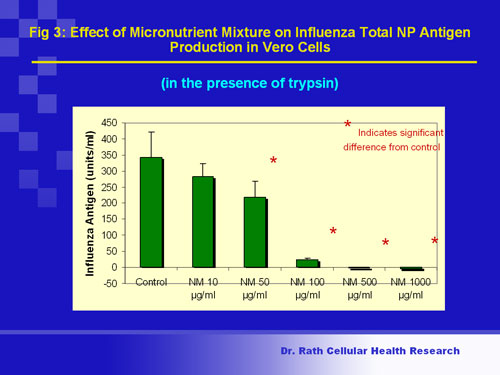
Fig. 3
Figure 3. Viral growth (multiplication): The central core of a flu virus contains genetic material called RNA that is packed together with a protein called NP (nuclear protein). The viral RNA contains all genes necessary for viral reproduction and survival. In cells infected with the flu virus, a decrease in NP production indicates that viral multiplication has been lowered. Figure 3 shows that application of the nutrient mixture to infected kidney cells immediately after infection resulted in dose-dependent suppression of NP production, with complete inhibition occurring at the highest concentrations tested.
Conclusion: Micronutrients can completely block the multiplication of human influenza A (H1N1) virus in infected kidney cells.
In addition, in the presence of these micronutrients, cells infected with the virus had reduced ability to secrete metalloproteinases to their surroundings. This indicates a decreased ability of viruses to spread and infect other cells. (Fig 4).
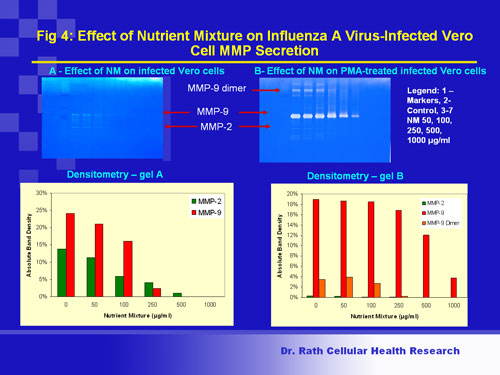
Fig 4
Figure 4. Viral spread: Viruses spread in the tissue and reach blood vessels by disrupting the connective tissue barrier made of collagen. The influenza virus not only infects pulmonary areas but also manifests in extra-pulmonary areas, which require basement membrane disruption by collagen-digesting enzymes called matrix metalloproteinases (MMP-2 and MMP-9). By limiting secretion of these enzymes, virus spread in tissues can be curbed. Influenza A viral infection was accompanied by cellular secretion of MMP-2 and MMP-9; the levels of MMP-9 were further enhanced upon exposure to the chemical inducer TPA (see Fig. 4). Treatment of infected cells with the nutrient mixture resulted in dose-dependent inhibition of both MMP-2 and MMP-9 in un-induced cells as well as that of MMP-9 in TPA-induced cells.
Conclusion: Nutrient mixture can inhibit secretion of collagen-digesting enzymes MMP2 and MMP9 that are linked to viral spread in cells infected by influenza A (H1N1) virus.
Suppression of viral replication was enhanced when viruses were exposed to this nutrient mixture prior infecting cells. These inhibitory effects of the nutrient combination were virus specific and not due to non-specific effects on cellular metabolism.
In conclusion, these pre-clinical results that we have obtained have important implications for the development of natural therapies to control influenza infection.
They have shown that a non-toxic micronutrient mixture has the potential to inhibit viral enzyme (neuraminidase activity) in cell-free virus particles, suppress viral nucleoprotein production in host cells, and reduce the secretion of invasive cellular metalloproteinases linked to virus spread.



