Translations: Deutsch, Nederlands
- Have any questions? Contact us!
- info@dr-rath-foundation.org
U.S. Government-Funded Study Concludes Risks Of COVID-19 Vaccines Not Being Adequately Disclosed
March 12, 2021
Doctors and Scientists Write to European Medicines Agency Warning of COVID-19 Vaccine Dangers
March 17, 2021COVID-19 VACCINES: Breakthrough Approach Or Unanticipated Health Risk?
Authored by Aleksandra Niedzwiecki, Ph.D. and Matthias Rath, M.D.
Dr. Rath Research Institute, San Jose, CA 95138, USA
The coronavirus pandemic has been surrounded by fears of contracting the virus. These were facilitated further by inconsistent and often contradictory information coming from the media, as well as from various scientific and political authorities. The introduction of a new generation of anti-COVID-19 vaccines at the end of 2020, with claimed efficacy in curbing the pandemic, and with strong media support, created high hopes and has been driving the demands for vaccination. At the same time, with these new types of vaccines still being at the experimental stage, questions about their efficacy against mutated forms of viruses, and a risk of unanticipated long-term side effects, are fueling reluctancy towards their acceptance. These concerns are not being dispersed by aggressive media campaigns or silencing critical voices of vaccination, but rather the opposite. Avoiding ‘difficult questions’ generates additional concerns and, according to the opinion polls, about 40% of Americans are still reluctant to take the vaccine.
Here we present a short overview of COVID-19 vaccines, including their mechanisms of action and safety aspects.
The road to genetically engineered vaccines (GEVs)
Vaccines have successfully been used in preventing various infective diseases for over two centuries. The first successful vaccine was developed against smallpox by Edward Jenner in 1796. Since then, the development of vaccines has been based on the use of attenuated viruses or bacteria, or on specific proteins generated by the pathogens. Injection of these can teach the immune system to recognize these antigens and mount an effective immune response, resulting in protection against a real infection. The development, production, and regulatory approval for this type of vaccine takes a long time (about 10 to 15 years) and is associated with high cost.
Since maximizing profits is the basis of the pharmaceutical business, pharmaceutical companies and their sponsored research have been experimenting with other technologies that would result in producing vaccines more quickly and at low cost. These new vaccines introduce the genetic code of pathogens to our cells. So instead of providing the infectious agents from outside like in conventional vaccines, the inserted genetic information triggers the production of viral or other pathogen proteins by our own body cells. This technology has been tested in animal models and recently applied in only a few human vaccines (e.g., Ebola, HIV, SARS, MERS, and others) with mixed results.
In 2012 the US Defense Advanced Research Projects Agency (DARPA) began funding groups at Novartis, Pfizer, AstraZeneca, Sanofi Pasteur and elsewhere to intensify work on RNA-encoded vaccines and therapeutics. The emergence of COVID-19 in 2019 subsequently triggered an urgent need for effective vaccines to control the rapidly expanding pandemic. In May 2020, the Trump administration initiated a public-private partnership – Operation Warp Speed – to coordinate efforts in the development, production, and distribution of vaccines and diagnostics against COVID-19. In December 2020, anti-COVID 19 vaccines produced by Pfizer/BioNTech and Moderna Therapeutics were approved. Over 17 million doses were distributed by the beginning of January 2021. Other countries run their own vaccine projects. Currently there are several anti-COVID vaccines developed and produced in the UK, Germany, Russia, China, and other countries.
How anti-COVID-19 genetically engineered vaccines work
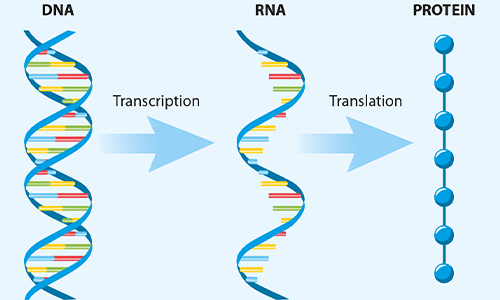
FIG 1. The key steps in protein synthesis in the cells
The vaccines produced by Pfizer, Moderna, AstraZeneca/University of Oxford and Janssen (a Johnson & Johnson company) apply genetically altered DNA or RNA to prompt host cells to make proteins specific to a pathogen. They use our cells’ protein synthesis machinery, which takes genetic information in the DNA as a template to transcribe it into messenger RNA (mRNA) molecules. These are then translated by the cells’ ribosome system to produce proteins. The code (sequence) for each protein a cell produces is encrypted both in DNA (as a blueprint) and mRNA (as a working copy).
How mRNA-based vaccines work
The vaccines developed by Pfizer/BioNTech and Moderna Therapeutics contain an mRNA strand that contains the code for a SARS-CoV-2 virus surface protein (known as a ‘spike’). This spike binds to the specific docking stations on a target cell membrane (the ACE2 receptor) that are necessary to initiate viral entry and infection. Therefore, antibodies directed against the spike protein can prevent the binding of the virus to cells, and consequently its infectivity.
The mRNA vaccines contain an mRNA strand with spike protein information. This is encapsulated into lipid nanoparticles (LNPs) that allow RNA to be transported through the cellular membrane and, at the same time, protect it from degradation by cellular enzymes. When injected,the mRNA in a vaccine enters the cells. Ribosomes subsequently translate it into the viral protein, producing multiple copies which are displayed on the cell surface. The body’s immune system recognizes this viral spike protein as foreign and develops antibodies and other immunity weapons in order to fight it.
The Pfizer and Moderna vaccines use so-called ‘non-replicating mRNA’ which, in addition to the code for the spike protein, have additional sequences at both ends to assure proper RNA processing. According to the manufacturers, once the viral antigen is produced the mRNA is degraded and cleared.
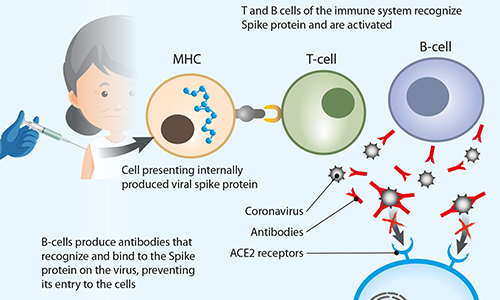
FIG 2. Schematic representation of how a COVID-19 vaccine triggers an immune system response
There are also other types of RNA vaccines under development, such as so-called self-replicating mRNA. In these vaccines the pathogen-mRNA strand is packaged with additional viral replication machinery that enables the host cells to amplify viral RNA and produce an abundant amount of viral protein. This means that greater quantities of the antigen are made from a smaller amount of vaccine for a more robust immune response.
What do COVID-19 mRNA vaccines contain?
Each dose of Pfizer’s product contains 30 micrograms of vaccine. Moderna produces a much larger dose of vaccine (100 micrograms) and yet it does not provide better results. At the request of the US government, Moderna has been testing its vaccine to determine whether it could lower the dosage without eroding the protection.
According to information provided by Moderna, its COVID-19 vaccine contains the following ingredients: messenger ribonucleic acid (mRNA); lipids (SM-102, polyethylene glycol [PEG] 2000, dimyristoyl glycerol [DMG], cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]); tromethamine; tromethamine hydrochloride; acetic acid; sodium acetate; and sucrose.
In addition to the mRNA molecule, Pfizer’s vaccine also contains lipid components ((4-hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3-phosphocholine, cholesterol)); salts (potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate); and basic table sugar
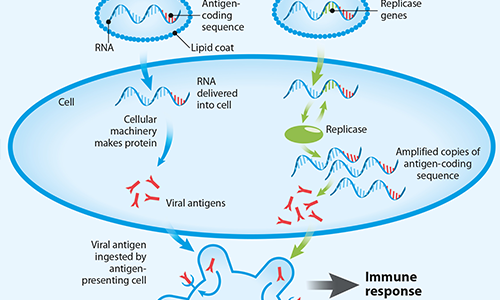
FIG 3. Different types of mRNA-based vaccines
to help the vaccine molecules maintain their shape during freezing.
RNA vaccines, like conventional vaccines, need to be frozen or refrigerated. This has been complicating their distribution. Pfizer’s vaccine must be stored in an ultra-cold freezer at temperatures between -80ºC and -60ºC (-112ºF to ‑76ºF) wherein it retains activity for up to 6 months. According to more recent information, this vaccine can also be stored at standard freezer temperatures of -25°C to -15°C (-13°F to 5°F) for a total of two weeks, or in normal refrigerator conditions for up to 5 days.
The Moderna vaccines are easier to transport and store as they can be kept for up to 30 days at normal refrigerator temperatures, and for up to six months in a freezer.
How DNA-based vaccines work
The Johnson & Johnson and AstraZeneca vaccines use a different approach known as a viral vectored vaccine. Viral vectors, such as the modified chimpanzee adenovirus (ChAdOx1) in the AstraZeneca vaccine and human adenovirus (AD26) in the Johnson & Johnson vaccine, come from a large family of viruses, some of which cause the common cold. The adenovirus DNA linked to viral genetic code (DNA) for the SARS-CoV2 spike protein is used to insert viral protein information into the cell nucleus. The cells use that code to make viral spike mRNA, and ultimately spike proteins. In the complex process of SARS-CoV-2 spike protein presentation by the cells, the viral protein becomes recognized by our body’s immune system as foreign, thus triggering an immune response.
Researchers believe that higher amounts of DNA within the range of 5 to 20 mg would need to be injected into an average-sized human to increase the immunogenicity of DNA-based vaccines. The Johnson & Johnson and AstraZeneca vaccines are single-dose products. Johnson & Johnson is also testing a two-dose regimen, with the shots given eight weeks apart. The results from this 30,000-person trial aren’t expected until sometime in May 2021.
The COVID-19 DNA-based Johnson & Johnson vaccine includes the following ingredients: recombinant, replication-incompetent DNA of adenovirus type 26 expressing the SARS-CoV-2 spike protein; citric acid monohydrate; trisodium citrate dihydrate; ethanol; 2-hydroxypropyl-β-cyclodextrin (HBCD); polysorbate-80; sodium chloride.
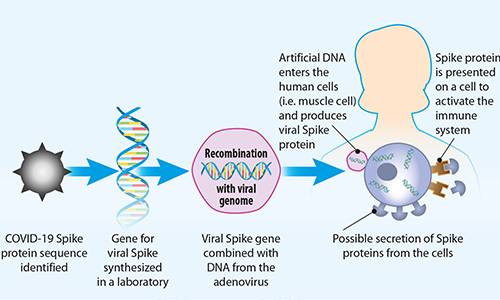
FIG 4. How DNA vaccines work
The Johnson & Johnson COVID-19 single-dose vaccine is estimated to remain stable for two years at -20°C (-4°F), and a maximum of three months with routine refrigeration at temperatures of 2 to 8°C (36-46°F). Work is ongoing to reliably produce vaccines that are more suitable for use in countries with limited or no refrigeration facilities.
Another challenge of DNA-based vaccines involves the optimization of transfection, which could be achieved through the incorporation of several parameters such as a hybrid viral/eukaryotic promotor or the optimization of antigen codons.
However, this has not impeded significant progress towards the use of this type of vaccine in humans, and they are being tested in various clinical trials.
Vaccine approval process
Current COVID‑19 vaccines have not been approved or licensed by the US Food and Drug Administration (FDA), but have instead been authorized only for emergency use to prevent Coronavirus Disease 2019 (COVID‑19) in individuals 18 years of age and older.
These vaccines have not undergone the same type of review as an FDA approved or cleared product. The emergency use authorization is allowed when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic, and that the known and potential benefits of the product outweigh the known and potential risks. All of these criteria must be met in order for the product to be used during the COVID-19 pandemic. Currently there is no FDA-approved vaccine to prevent COVID‑19.
The emergency use authorization for the Janssen COVID-19 vaccine is in effect for the duration of the COVID-19 declaration justifying emergency use of these products, unless terminated or revoked (after which the products may no longer be used).
How effective are COVID-19 vaccines in a population?
The term ‘efficacy’ refers to the vaccine’s performance under ideal and controlled circumstances, such as in clinical trials. The term ‘effectiveness’ refers to its performance in real-world situations.
Based on clinical trials, the manufacturers of both RNA- and DNA-based vaccines claim a high efficacy in preventing COVID-19 infections (about 90%) in people 16 years old and older. A recent large observational study conducted in Israel estimates that the Pfizer COVID-19 vaccine is 46% effective at preventing infection 14 to 20 days after the first dose, and 92% effective 7 days after the second dose. The pooled results from trials with the AstraZeneca vaccine claim an overall vaccine efficacy of 66.7% against symptomatic COVID-19 14 days after the second dose.
There is no evidence that any of the current COVID-19 vaccines can completely stop or significantly lessen the chances of people from being infected, or that they stop transmission of the virus if vaccinated people become infected. Early evidence suggests that in some cases, when people are infected after being fully vaccinated, they experience a milder course of illness than they otherwise would have done.
Earlier observed sex differences in immune response and hormonal status suggest that vaccines and therapies increasing T cell immune response to SARS-CoV-2 might be appropriate for male patients, with those dampening the innate immune activation in the early stage of the disease appropriate for females (Takahashi T et al, 2020).
How effective are COVID-19 vaccines against mutated forms of coronavirus?
Since the emergence of the pandemic, the appearance of SARS‐CoV‐2 mutants has been a concern. This process of evolution is continuous and allows the virus to select changes fast that make it grow more efficiently. Any change that gives the virus’s descendants a competitive growth advantage will be favored and begin to outgrow the original parent virus. Thus, it is important to monitor viruses for new mutations that could make them more deadly, more transmissible, or both.
As early as February 2020, a substitution in the spike protein of SARS‐CoV‐2 was detected and named D614G variant (Korber B et al., 2020). Four months later, this variant – having increased infectivity, although still with a comparable disease severity to its wild‐type strain – had become prominent globally. More recently, another more transmissible strain variant (B.1.1.7.) has surfaced in United Kingdom. The B.1.1.7. variant has 17 mutations, 8 of which are in the spike protein. One of the spike mutations, N501Y, has also been found on another variant of the virus isolated in South Africa (Reardon S, 2020). At the time of writing, the emerging evidence suggests that some of these recent variants can evade both natural- and vaccine‐induced immunity when tested in laboratory test tubes. It remains to be fully established whether and to what extent this may lead to reduced vaccine efficacy (Callaway E, 2021).
Current COVID-19 vaccines induce the immune system to produce antibodies that recognize and target the spike protein on the virus, which is essential for its binding to ACE2 receptors and invading human cells. With multiple alterations in the spike protein sequence, vaccines designed for the original strain of the virus may no longer produce a strong immune response against new variant viruses.
Detecting new mutations is a logistical challenge as it requires sequencing viruses from infected patients’ samples to detect variants. Current efficacy in detecting viral mutations is inadequate. The US, despite having the largest number of infections, is in 43rd place in sequencing for the presence of coronavirus mutants. South Africa is in 42nd place. The UK stands much better, being in 8th place, with 17% of patient samples tested for eventual mutations. The leading nations in this respect are Australia and New Zealand.
Safety aspects of covid-19 vaccines: knowns, known unknowns, and unknown unknowns
Since the public has been exposed to COVID-19 vaccination for only a few months, the long-term side effects are not yet fully known. It is not clearly explained by the manufacturers how long the spike protein production will last after the immunization. The long-term aggravation of the immune system by viral spike protein may increase the risk of an autoimmune response. The clinical trials and public vaccinations conducted so far were only able to unravel health issues that developed within a short time. However, at the time of writing, the governments of Denmark, Italy, and Austria have suspended use of the AstraZeneca vaccine following reports of deadly blood clots in vaccinated patients, in addition to reports of a death and an illness among vaccine recipients. These countries are joined by Estonia, Lithuania, Luxembourg, Iceland, and Latvia in suspending vaccines from this manufacturer. Of course, such incidents are vigorously denied as coincidental and fought against by vaccine manufacturers defending their stock prices and revenues.
- Known short-term side effects
Thus far, the most commonly reported side effects of the Pfizer vaccine, typically lasting several days, were pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, and fever. More people (about 20-40%) experienced these side effects after the second dose than after the first dose. According to reports submitted to the FDA, about 31% of people between the ages of 18 and 55 receiving the second dose of the Pfizer vaccine reported fever. With the Moderna vaccine, about 1% of those aged 18 to 64 reported fever after the first dose, while 17 percent reported getting a fever after the second dose.
Side effects are typically more pronounced among younger people than older people, likely because their immune systems are more robust.
The most recent data from the US Centers for Disease Control and Prevention (CDC) show that serious side effects, such as anaphylactic allergic reactions, occur at a rate of about 2.5 cases per one million doses given of the Moderna vaccine, and 4.7 cases per million doses of the Pfizer product. Many of the people who have developed anaphylaxis have a history of severe allergies, with some having had previous episodes of anaphylaxis.
Severe reactions, although transient, do seem to be more common with COVID-19 shots than with other immunizations — more than 80% of people who received the Moderna vaccine in clinical trials had some type of systemic reaction to the shot, with bouts of fatigue, muscle pain, and other issues that often proved briefly debilitating.
Contaminants originated in the vaccine synthesis process and the nanoparticle delivery system are said to be two of the main sources of adverse reactions. For these reasons, vaccine manufacturers often administer lower doses to limit a person’s exposure to them. With mRNA vaccines, however, lower doses mean lower potency.
- Potential long-term side effects of GEV vaccines: Known unknowns
Potential risks associated with mRNA-based vaccines. The mRNA strand in the vaccine may elicit immune reactions that can include local and systemic inflammation, autoimmune reactions from stimulation of auto-reactive antibodies, the biodistribution and persistence of expressed immunogen, and potential toxic effects of any non-native nucleotides and delivery system components. A possible concern could be that some mRNA-based vaccine platforms induce potent type I interferon responses (Pepini, T. et al. 2017), which have been associated not only with inflammation but also potentially with autoimmunity (Theofilopoulos A. N. et al., 2005, Nestle F. O. et al. 2005).
Another potential safety issue could derive from the presence of extracellular RNA during mRNA vaccination. Extracellular naked RNA has been shown to increase the permeability of tightly packed endothelial cells and may thus contribute to oedema (Fischer S. et al. 2007).
Another study showed that extracellular RNA promoted blood coagulation and pathological thrombus formation (Kannemeier C. et al. 2007). This would confirm the recent case of a deadly blood clot reported with the AstraZeneca vaccine.
It should also be noted that patients who have severe reactions to the infection might be more at risk for adverse reactions to the vaccine (e.g., cytokine storm), which may be provoked by foreign mRNA strands.
Conversely, individuals with asymptomatic presentation might be potential vaccine non-responders. Thus, the genetic markers identified as predictors of COVID-19 severity should be taken into account during the development and administration of vaccines.
Potential risks of DNA-based vaccines. Many aspects of the immune response generated by DNA vaccines are still not understood. This technique carries a risk of affecting genes controlling cell growth if the vector is incorporated in a critical part of the host DNA code, which may result in serious consequences.
Other major safety concerns in DNA-based vaccines relate to the possibility of inducing antibody production against DNA resulting in autoimmune diseases, developing tolerance to the viral protein (antigen) introduced in the DNA, as well as the negative health impact of novel molecular adjuvants.
Further safety concerns include the possible spread of genetic material to the environment via the potential transformation of the environmental microflora with only a few copies of complete or fragmented plasmid.
Other risk concerns. Since COVID-19 vaccines have been approved without extensive long-term safety testing, they can’t exclude any unanticipated risks. Examples include the following:
- The risks of inducing prion-based diseases by activating intrinsic proteins to form prions. These misfolded proteins cause several fatal and transmissible neurodegenerative diseases, including Mad Cow Disease in cattle and Creutzfeldt-Jakob disease in humans. Prions are also implicated in Alzheimer’s disease and Amyotrophic Lateral Sclerosis (ALS). A report originating from the Human Microbiology Institute labs in New York, funded by the pharmaceutical firm Johnson & Johnson, indicates the presence of prion-related sequences in the COVID-19 spike which were not found in other coronaviruses. It is anticipated that these sequences may be present in the spike protein code in mRNA or DNA vaccines.
- There are no publicly available data on how long the viral RNA is translated in a vaccine recipient and how long the spike protein will be present on a host cell. This novel spike protein may become a receptor for other as yet unknown infectious agents. It can also be secreted to the cell’s external environment with unpredictable consequences.
- Some researchers are concerned with a potential of affecting genetic diversity by placing an identical viral spike protein on the cells of everyone in the global population. This would create an identical potential receptor to be targeted by other infectious agents that are as yet unknown. In such a situation, everyone in the population would have become potentially susceptible to pathogen binding with the same agent.
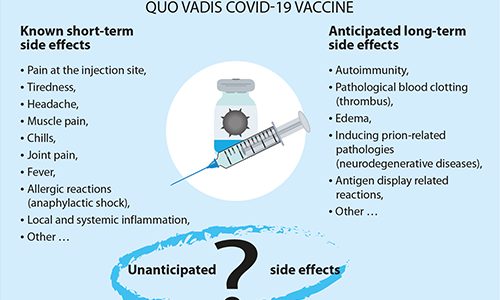
Unanticipated side effects of GEV vaccines: unknown unknowns. In addition to commonly observed side effects, the risks of allergic reactions and other predictable risks, we cannot exclude the possibility that other serious and unexpected effects may occur years later after hundreds of millions of people of different ages, races, ethnicities, and health conditions have been exposed to COVID-19 vaccines. The identification of these effects and their linkage to the vaccination represent a real logistical challenge. This experimental technology therefore calls for effective post-marketing surveillance.
This review represents the first part in a series of topics related to COVID-19 vaccines. The next part coming shortly will discuss the role of micronutrients as an effective first defense against infections, and their role as natural ‘vaccines’ and immune system boosters.
References
Callaway E. Fast‐spreading COVID variant can elude immune responses Nature. 2021;589:m4944.500–501.
Fischer, S. et al. Extracellular RNA mediates endothelial-cell permeability via vascular endothelial growth factor. Blood 2007; 110, 2457–2465.
Kannemeier, C. et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc. Natl Acad. Sci. USA 2007; 104, 6388–6393
Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 Virus. Cell. 2020;182(4):812‐827.
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005; 202, 135
Pepini, T. et al. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J. Immunol. 2017;198, 4012–4024.
Reardon S, The U.K. Coronavirus Mutation Is Worrying but Not Terrifying. Scientific American. December 24,2020.
Takahashi T., Yale IMPACT Research Team. Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., Silva J., Mao T., Oh J.E., et al. Sex Differences in Immune Responses That Underlie COVID-19 Disease Outcomes. Nature. 2020:1–9. doi: 10.1038/s41586-020-2700-3
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 2005; 23, 307–336.



