- Have any questions? Contact us!
- info@dr-rath-foundation.org

Resveratrol Supplements May Help Astronauts Preserve Bone And Muscle Mass On Mars Missions
July 31, 2019
Study Finds UK’s Intake Of Key Vitamins And Minerals Has Plummeted Over Last 20 Years
August 1, 2019New Independent Analysis Raises Serious Doubts Over Safety Of Artificial Sweetener Aspartame
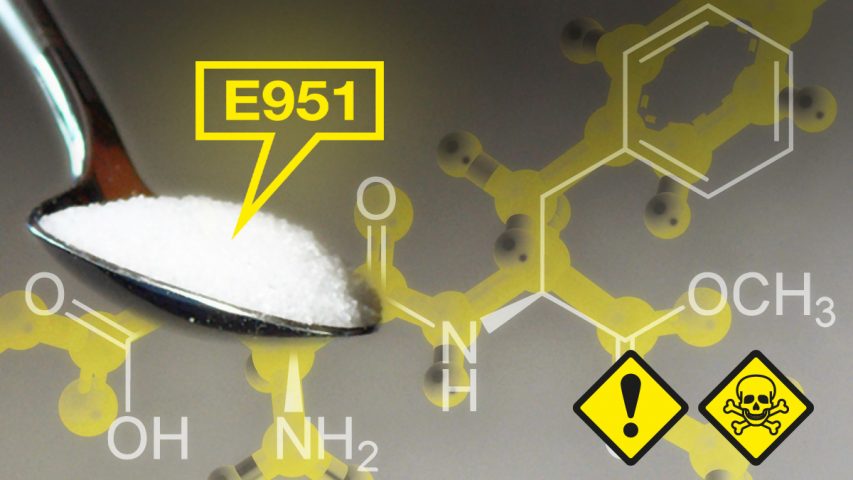
A new independent analysis of the European Food Safety Authority (EFSA) risk assessment of aspartame, the world’s most widely used artificial sweetener, has raised serious doubts over the chemical’s safety. Published by scientists from the University of Sussex in the UK, the analysis points out that, of 73 studies indicating aspartame could be harmful, EFSA’s assessment discounted the results of every single one. Conversely, EFSA categorized as reliable 84 percent of studies claiming there was no evidence of harm from the sweetener. Given this degree of bias, and the shortcomings of all previous official risk assessments of the chemical, the UK scientists argue that it would be premature to conclude it is safe.
Published in December 2013, EFSA’s risk assessment of aspartame was provided by its so-called ‘Panel on Food Additives and Nutrient Sources Added to Food’. After conducting a forensic examination of the assessment, UK scientists Professor Erik Millstone and Dr. Elisabeth Dawson discovered it contained numerous flaws. These included that it breached EFSA’s own guidelines on risk assessment transparency; that it adopted a low hurdle for the acceptability of pro-aspartame studies, including those previously dubbed “woefully inadequate” and “worthless” by experts; and that it applied unreachably high hurdles for studies indicating the chemical wasn’t safe. Tellingly, many of the 73 studies discounted by EFSA were far more reliable than most of those claiming there was no indication of risk.
Previously, in 2013, Professor Millstone had produced a 30-document dossier detailing the inadequacy of 15 early pivotal studies on aspartame. However, EFSA failed to pass this dossier to its scientific advisors. Commenting on his latest findings, Professor Millstone said: “It is clear from this research that the EFSA scientists failed to acknowledge numerous inadequacies in the reassuring studies, but instead picked up on tiny imperfections in all the studies providing evidence that aspartame may be unsafe.”
He added that: “In my opinion, based on this research, the question of whether commercial conflicts of interest may have affected the panel’s report can never be adequately ruled out because all meetings took place behind closed doors.”
On the basis of his new research, Professor Millstone is now calling for the suspension of authorization to sell or use aspartame in Europe pending an independent and thorough re-examination of all the relevant evidence. He is also advocating a radical overhaul of European food safety processes, including an end to behind-closed-door discussions.
The world’s most controversial food additive

U.S. FDA Commissioner Arthur Hayes authorized aspartame in 1981 against the advice of his own toxicologists and an FDA review board
Food and Drug Administration [Public domain], via Wikimedia Commons
The decision of newly appointed FDA Commissioner Arthur Hayes to authorize aspartame in 1981 was not only taken against the advice of his own toxicologists, but also that of an FDA review board. Also worthy of note is that following the Ronald Reagan administration gaining control of the White House in January 1981, Donald Rumsfeld, who at the time served as president and CEO of Searle, is said to have accepted the post of Reagan’s ‘Special Presidential Envoy to the Middle East’ in exchange for a promise that the new administration would get the chemical onto the market. Rumsfeld had previously reportedly stated that he would “call in his markers” to obtain a license for it.
Today, aspartame is found in thousands of food products worldwide, including diet soft drinks. With research clearly indicating the chemical has carcinogenic effects and that it can cause neurological and behavioral disturbances, the calls for it to be removed from the market will inevitably only grow louder. The longer that governments and regulators fail to act on this, the greater will be the impression that they see the profits of multinational pharmaceutical and chemical companies as more important than the health of ordinary citizens. Given that public trust in governments and regulators has been falling for years now in many countries, it is time for them to be reminded whose interests they are meant to serve.



